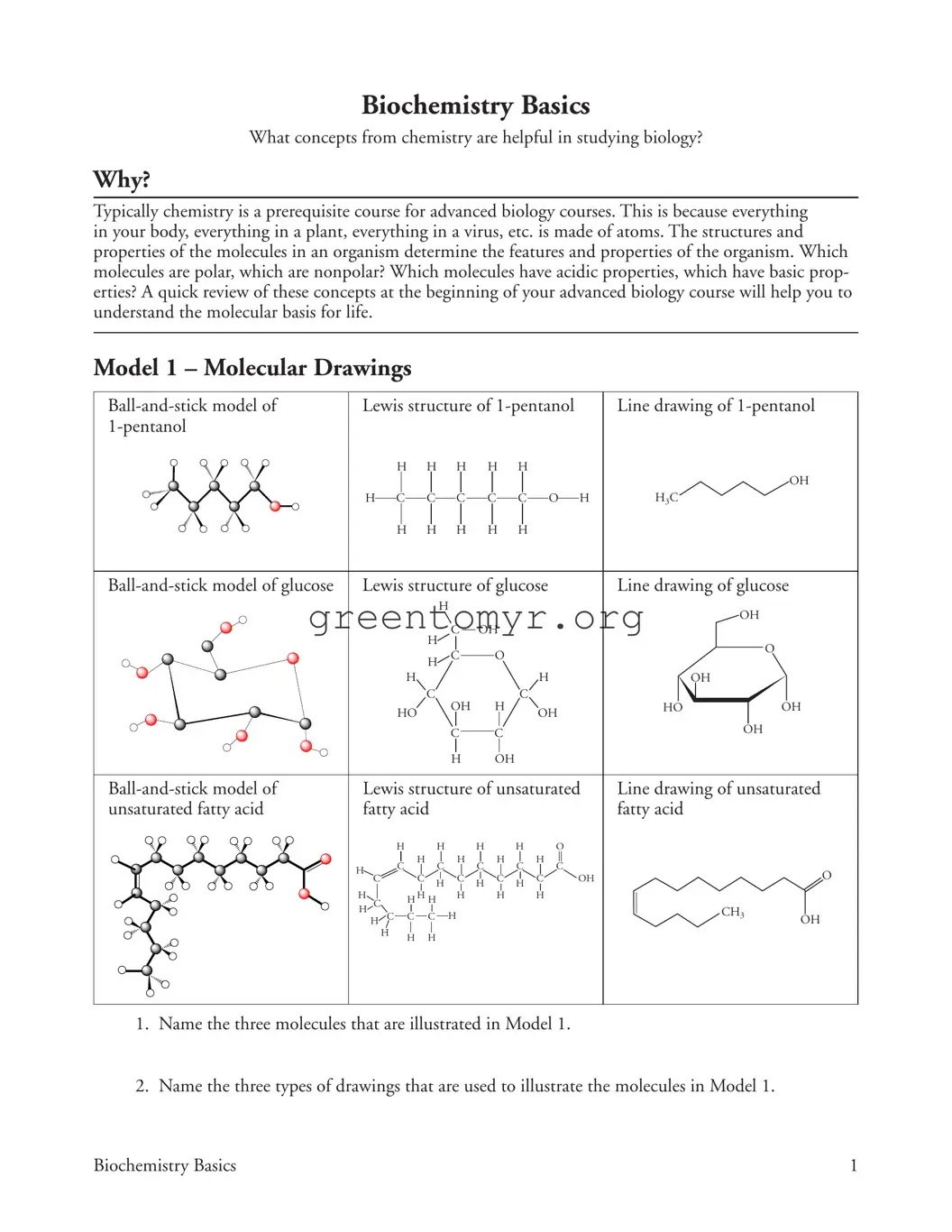
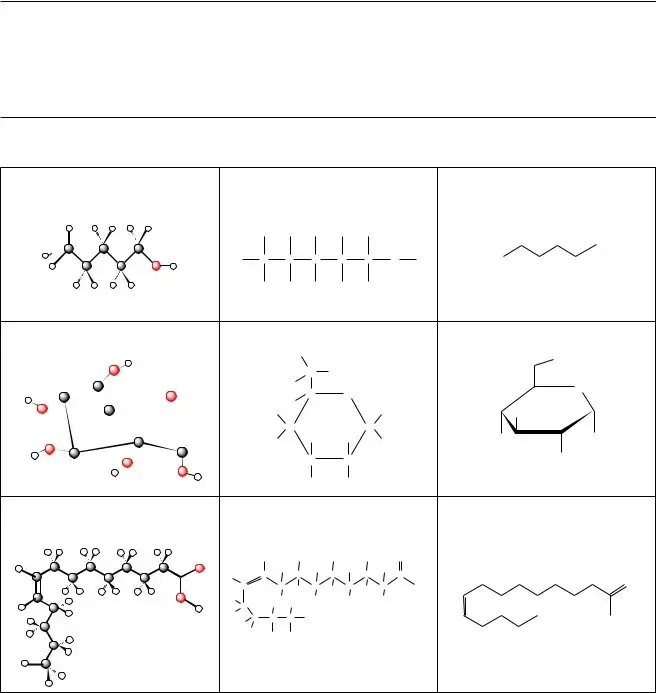
Filling out the Biochemistry Basics form requires attention to detail, and many individuals make common mistakes that can lead to confusion or errors in their submissions. One frequent issue is the omission of specific molecule names. For example, the form asks for three molecules illustrated in Model 1. Failing to name these correctly can result in an incomplete or inaccurate response.
Another mistake involves misunderstanding the types of drawings used in the model. Applicants should carefully identify the "ball-and-stick model," "Lewis structure," and "line drawing." Mislabeling these will hinder clarity and demonstrate a lack of comprehension of molecular representations.
When addressing the number of bonds formed by different atoms, individuals often miscount. For carbon, hydrogen, and oxygen, it is crucial to recognize typical bonding patterns. Making a mistake here not only affects the accuracy of the answer but also reflects a fundamental misunderstanding of chemical principles.
People also tend to overlook the question about accurate images of molecular shapes. The form asks for justification regarding which type of drawing provides a clearer representation of molecular structure. Insufficient reasoning can lead to a less persuasive argument, reducing the quality of the response.
In section five, individuals may skip crucial elements in their answers regarding missing symbols or atoms. This oversight can lead to incomplete or incorrect representations of molecular structures, which is a critical aspect of understanding organic compounds.
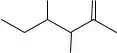
Moreover, when asked to redraw the structure of isoleucine, some fail to accurately locate and represent carbon and hydrogen atoms. This step tests understanding and drawing skills; inaccuracies can detract from overall performance on the form.
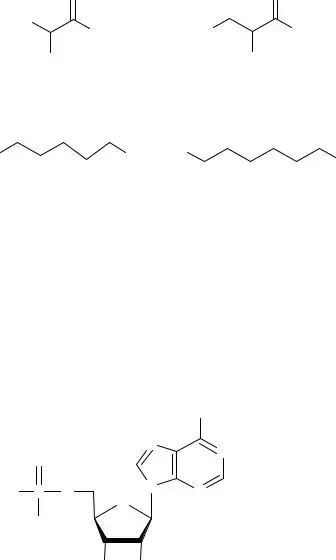
Applicants sometimes struggle with the drawing of isopropyl alcohol due to its structural nuances. Neglecting to represent it using all three drawing methods can showcase a lack of versatility in visual representations of molecules, which is a significant part of biochemistry.
When asked which type of drawing would be the easiest for writing a chemical formula, many fail to articulate their reasoning effectively. The effectiveness of their drawn representation plays an important role in justifying their choice, and without proper explanation, their response may seem incomplete.
In discussing the advantages of line drawings, people tend to either provide vague responses or neglect to compare these to the other drawing types. A well-rounded understanding of the advantages is essential in showcasing one's ability to communicate scientific concepts.
Lastly, when it comes to understanding polar and nonpolar molecules, individuals frequently miss crucial details about atom presence in the molecules. Ignoring key distinctions leads to unclear answers that may misinterpret fundamental chemical properties. Overall, these common mistakes can undermine the quality of responses on the Biochemistry Basics form, thereby impacting the overall understanding of biochemistry concepts.

 H
H